Search
Recent comments
- grab....
52 min 55 sec ago - enlightenment....
2 hours 3 min ago - victory....
2 hours 9 min ago - unrest....
3 hours 7 min ago - disinformation...
10 hours 52 min ago - poisoned dart?....
14 hours 11 min ago - narrative control....
18 hours 18 min ago - chabad....
23 hours 34 min ago - back to the kitchen....
23 hours 36 min ago - loneliness....
1 day 1 hour ago
Democracy Links
Member's Off-site Blogs
on global warming...
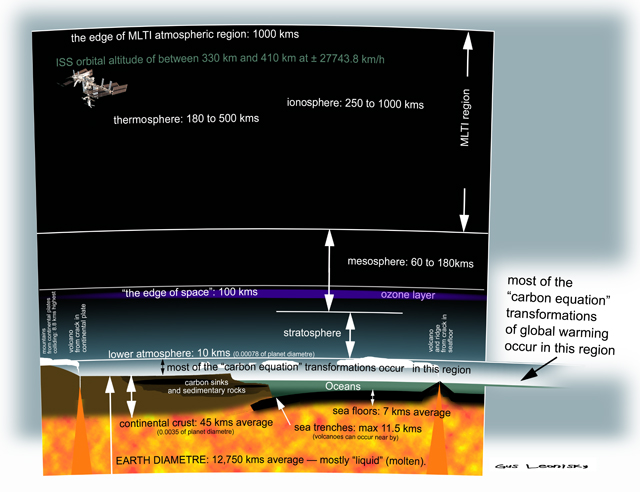
- By Gus Leonisky at 28 Jun 2012 - 12:12pm
- Gus Leonisky's blog
- Login or register to post comments
explaining the way it works...
Over the next few weeks I will post on this line of blogs diagrams that will explain how GLOBAL WARMING works...
Anyone intelligent knows the process is complicated.
There are many influences on global climate including latitude and the layering of the atmosphere... These layers are not defined exclusively though they have different "qualities". These layers overlap and interact on fluctuating blurred edges... Thus someone might place the thickness of the "lower atmosphere" at 10 kms while others might place it at 11 kms... This makes nil difference to the understanding of the processes.
The altitude of breathable atmosphere stops at 8,000 metres for heroes but for most of us, mere mortals, we gasp for oxygen at 5,000 metres...
Some factors are important:
atmospheric pressure
altitude
humidity
temperature
air density
ionisation
earth spin axis wobble
sun flares
sun spots
volcanoes
clouds
water vapour
gaseous mix of the atmosphere
dew point
wind
jet streams
life
oceans
carbon sinks
etc....
the lucky planet...
When comparing the Earth to other planets in the solar system, one has to wonder about our luck... The Earth is a bloody lucky planet in a system of solar winds, transformation of gaseous atmosphere and surface temperature while travelling in a very cold cosmos...
For example the surface of Mercury — the closest planet to the sun — is around 220 degrees Celsius on average... This of course changes with the day (400+ degrees Celsius at points closest to the sun) and night (-180 degree Celsius) of Mercury— which for all intent and purposes takes 176 Earth-day for the switch to be complete while the planet only takes 88 Earth days to complete its own orbit around the sun...
Mercury's atmosphere is very very thin, probably stripped away and added to — by solar winds — of mostly atomic helium and hydrogen...
The Atmosphere on Venus is nearly 100 times thicker than that of Earth. One would expect that Venus being quite further away from the sun than Mercury, its surface temperature would be (calculated at) 25 per cent that of Mercury. But the "Venerean" (of Venus) atmosphere being mostly CO2 creates a strong "greenhouse effect" that keeps the temperature at about 480 degrees Celsius — day and night and in the polar regions as well... The "Venerean" atmosphere absorbs heat from the sun and the equilibrium between sidereal heat loss and heat retained stands at that warm 480 degrees Celsius on the surface of Venus...
In comparison, the Thermosphere — a very very low density layer of the Earth's atmosphere — is above 1000 degrees Celsius during the Earth day and varies during the night between 0 to -100 degrees Celsius...
On Mars, a planet the atmosphere of which is quite thin and made mostly of CO2 (95 %), the barometric pressure is a third of that on Earth, while the barometric pressure on Venus is nearly 100 times the pressure on Earth (as if 1000 metres below the sea). The surface temperature on Mars hover around -60 degrees Celsius. One could assume that without the thin atmosphere, the surface temperature of Mars could be a bit hotter during the day and far cooler during the Martian nights...
-------------------
Back to the LUCKY PLANET — THE EARTH
About 75 per cent of the gases in the atmosphere are contained in the lower part — ONLY 10,000 to 12,000 metres thick — the Troposphere... We live —with other creatures, from plants to animals — in the bottom part (between 0 to 5,000 metres depending on countries) of this very thin layer of gases, above a molten ball of hot lava with a very very thin crust...
The air pressure and the air temperature decrease with altitude and at about 5,000 metres we gasp for air... At 10,000 metres, the temperature is about minus 50 degrees Celsius. Between 10,000 and 12,000 metres, there is a thin layer of the atmosphere we call the Tropopause, where strong jet streams occur blowing eastward... Above 12,000 the temperature increases gradually until it reaches the Thermosphere where temperature increases rapidly.
The "average" temperature of this planet's SURFACE is 15 degrees Celsius, but it varies greatly with latitudes, and day and night, though the atmosphere and the oceans provide a "temperature drop cushioning effect" which is part of the "greenhouse effect" which in fact should be called THE "insulation effect" of the atmosphere... Without the atmosphere, the temperature during the day would possibly go above 100 degrees Celsius and below minus fifty during the nights...
The average temperature of the Earth's SURFACE has changed over al periods of geological time, due to various factors — including cosmic Bolides (meteorites and comets) hitting the Earth AND CONTINENTAL DRIFT. Thus there has been periods of massive glaciation, of small glaciation and of no glaciation at all (with "warmish" polar temperatures). Presently, we are in a small glaciation event with variation-extent from the last Ice Age (surface temperature cooler than present by 4 to 6 degrees Celsius 12,000 years ago) to now. I consider the last ice age to have been a small ice age compared to some earlier in the Earth's 4 billion years history.
We can say with confidence that the atmosphere is our insulating blanket... IT'S A VERY COMPLEX REACTIVE BLANKET. THE SUN and LIFE on Earth play important roles of influence on this blanket.
Other influences include VOLCANOES, the earth axis wobbles ( Chandler wobble) and orbit wobble (Milankovitch cycles), gravity, and of course the GASEOUS COMPOSITION of the atmosphere itself.
Happy breathing...
Next: The gaseous mix...
the gaseous mix...
This article mostly adapted from : http://en.wikipedia.org/wiki/Atmosphere_of_Earth and various other sources
The atmosphere of Earth is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention (insulation effect), and reducing temperature extremes between day and night (the diurnal temperature variation).
Atmospheric stratification has distinct layers, each with specific characteristics such as temperature or composition. The atmosphere has a mass of about 5×1018 kg, 75 % of which is within about 11 km of the surface. The atmosphere becomes thinner and thinner with increasing altitude, with no definite boundary between the atmosphere and outer space. An altitude of 120 km is where atmospheric effects become noticeable during atmospheric reentry of spacecraft.
The Kármán line, at 100 km also is often regarded as the boundary between atmosphere and outer space. The line was named after Theodore von Kármán, (1881–1963) a Hungarian-American engineer and physicist who was in the fields of aeronautics and astronautics. He first calculated that around this altitude the Earth's atmosphere becomes too thin for aeronautical purposes because any vehicle at this altitude would have to travel faster than orbital velocity to climb further up. There is an abrupt increase in atmospheric temperature and interaction with solar radiation at this jucture.
Air is the name given to the atmosphere used in breathing and photosynthesis. Dry air contains roughly (by volume) 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.039% carbon dioxide, and small amounts of other gases. Air also contains a variable amount of water vapor, on average around 1%.
While air content and atmospheric pressure vary at different layers, air suitable for the survival of terrestrial plants and terrestrial animals is currently only known to be found in Earth's TROPOSPHERE and artificial atmospheres.
Gases IN DRY ATMOSPHERE:Nitrogen (N2) 780,840 ppmv (78.084%)
Oxygen (O2) 209,460 ppmv (20.946%)
Argon (Ar) 9,340 ppmv (0.9340%)
Carbon dioxide (CO2) 394.45 ppmv (0.039445%)
Neon (Ne) 8.18 ppmv (0.001818%)
Helium (He) 5.24 ppmv (0.000524%)
Methane (CH4) 1.79 ppmv (0.000179%)
Krypton (Kr) 1.14 ppmv (0.000114%)
Hydrogen (H2) 0.55 ppmv (0.000055%)
Nitrous oxide (N2O) 0.3 ppmv (0.00003%)
Carbon monoxide (CO) 0.1 ppmv (0.00001%)
Xenon (Xe) 0.09 ppmv (9×10−6%) (0.000009%)
Ozone (O3) 0.0 to 0.07 ppmv (0 to 7×10−6%)
Nitrogen dioxide (NO2) 0.02 ppmv (2×10−6%) (0.000002%)
Iodine (I2) 0.01 ppmv (1×10−6%) (0.000001%)
Ammonia (NH3) trace
Water vapor (H2O) 0.40% over full atmosphere, typically 1%-4% at surface
Principal layers
In general, air pressure and density decrease in the atmosphere as height increases. However, temperature has a more complicated profile with altitude. Because the general pattern of this profile is constant and recognizable through means such as balloon soundings, temperature provides a useful metric to distinguish between atmospheric layers. In this way, Earth's atmosphere can be divided into five main layers. From highest to lowest, these layers are:
Exosphere
The outermost layer of Earth's atmosphere extends from the exobase upward. It is mainly composed of hydrogen and helium. The particles are so far apart that they can travel hundreds of kilometers without colliding with one another. Since the particles rarely collide, the atmosphere no longer behaves like a fluid. These free-moving particles follow ballistic trajectories and may migrate into and out of the magnetosphere or the solar wind.
ThermosphereTemperature increases with height in the thermosphere from the mesopause up to the thermopause, then is constant with height. Unlike in the stratosphere, where the inversion is caused by absorption of radiation by ozone, in the thermosphere the inversion is a result of the extremely low density of molecules. The temperature of this layer can rise to 1,500 °C, though the gas molecules are so far apart that temperature in the usual sense is not well defined. The air is so rarefied that an individual molecule (of oxygen, for example) travels an average of 1 kilometer between collisions with other molecules.[3] The International Space Station orbits in this layer, between 320 and 380 km.
Mesosphere
The mesosphere extends from the stratopause to 80–85 km. It is the layer where most meteors burn up upon entering the atmosphere. Temperature decreases with height in the mesosphere. The mesopause, the temperature minimum that marks the top of the mesosphere, is the coldest place on Earth and has an average temperature around −85 °C. At the mesopause, temperatures may drop to −100 °C (Due to the cold temperature of the mesosphere, water vapor is frozen, forming ice clouds (or Noctilucent clouds). A type of lightning referred to as either sprites or ELVES, form many miles above thunderclouds in the troposphere.
StratosphereThe stratosphere extends from the tropopause to about 51 km. Temperature increases with height due to increased absorption of ultraviolet radiation by the ozone layer, which restricts turbulence and mixing. While the temperature may be −60 °C at the tropopause, the top of the stratosphere is much warmer, and may be near freezing[citation needed]. The stratopause, which is the boundary between the stratosphere and mesosphere, typically is at 50 to 55 km. The pressure here is 1/1000 sea level.
TroposphereThe troposphere begins at the surface and extends to between 9 km at the poles and 17 km at the equator, with some variation due to weather. The troposphere is mostly heated by transfer of energy from the surface, so on average the lowest part of the troposphere is warmest and temperature decreases with altitude. This promotes vertical mixing. The troposphere contains roughly 80% of the mass of the atmosphere. The tropopause is the boundary between the troposphere and stratosphere.
Other layers
Within the five principal layers determined by temperature are several layers determined by other properties:
The ozone layer is contained within the stratosphere. In this layer ozone concentrations are about 2 to 8 parts per million, which is much higher than in the lower atmosphere but still very small compared to the main components of the atmosphere. It is mainly located in the lower portion of the stratosphere from about 15–35 km, though the thickness varies seasonally and geographically. About 90% of the ozone in our atmosphere is contained in the stratosphere.The ionosphere, the part of the atmosphere that is ionized by solar radiation, stretches from 50 to 1,000 km and typically overlaps both the exosphere and the thermosphere. It forms the inner edge of the magnetosphere. It has practical importance because it influences, for example, radio propagation on the Earth. It is responsible for auroras.
The homosphere and heterosphere are defined by whether the atmospheric gases are well mixed. In the homosphere the chemical composition of the atmosphere does not depend on molecular weight because the gases are mixed by turbulence.
The homosphere includes the troposphere, stratosphere, and mesosphere. Above the turbopause at about 100 km, the composition varies with altitude. This is because the distance that particles can move without colliding with one another is large compared with the size of motions that cause mixing. This allows the gases to stratify by molecular weight, with the heavier ones such as oxygen and nitrogen present only near the bottom of the heterosphere. The upper part of the heterosphere is composed almost completely of hydrogen, the lightest element.
The planetary boundary layer is the part of the troposphere that is nearest the Earth's surface and is directly affected by it, mainly through turbulent diffusion. During the day the planetary boundary layer usually is well-mixed, while at night it becomes stably stratified with weak or intermittent mixing. The depth of the planetary boundary layer ranges from as little as about 100 m on clear, calm nights to 3000 m or more during the afternoon in dry regions.
The average temperature of the atmosphere at the surface of Earth is 14 °C or 15 °C depending on the reference.
Pressure and thickness
Total atmospheric mass is 5.1480×1018 kg, about 2.5% less than would be inferred from the average sea level pressure and the Earth's area of 51007.2 megahectares, this portion being displaced by the Earth's mountainous terrain. Atmospheric pressure is the total weight of the air above unit area at the point where the pressure is measured. Thus air pressure varies with location and weather.
In summary, the mass of the earth's atmosphere is distributed approximately as follows:[16]
50% is below 5.6 km.90% is below 16 km.
99.99997% is below 100 km, the Kármán line. By international convention, this marks the beginning of space where human travelers are considered astronauts.
The summit of Mt. Everest is at 8,848 m; commercial airliners typically cruise between 10 km and 13 km where the thinner air improves fuel economy; weather balloons reach 30.4 km and above; and the highest X-15 flight in 1963 reached 108.0 km.
Even above the Kármán line, significant atmospheric effects such as auroras still occur. Meteors begin to glow in this region though the larger ones may not burn up until they penetrate more deeply. The various layers of the earth's ionosphere, important to HF radio propagation, begin below 100 km and extend beyond 500 km.
Density of airThe density of air at sea level is about 1.2 kg/m3 (1.2 g/L). Density is not measured directly but is calculated from measurements of temperature, pressure and humidity using the equation of state for air (a form of the ideal gas law). Atmospheric density decreases as the altitude increases. This variation can be approximately modeled using the barometric formula. More sophisticated models are used to predict orbital decay of satellites.
The average mass of the atmosphere is about 5 quadrillion (5×1015) tonnes or 1/1,200,000 the mass of Earth.
Solar radiation (or sunlight) is the energy the Earth receives from the Sun. The Earth also emits radiation back into space, but at longer wavelengths that we cannot see. Part of the incoming and emitted radiation is absorbed or reflected by the atmosphere.
ScatteringWhen light passes through our atmosphere, photons interact with it through scattering. If the light does not interact with the atmosphere, it is called direct radiation and is what you see if you were to look directly at the Sun. Indirect radiation is light that has been scattered in the atmosphere. For example, on an overcast day when you cannot see your shadow there is no direct radiation reaching you, it has all been scattered. As another example, due to a phenomenon called Rayleigh scattering, shorter (blue) wavelengths scatter more easily than longer (red) wavelengths. This is why the sky looks blue; you are seeing scattered blue light. This is also why sunsets are red. Because the Sun is close to the horizon, the Sun's rays pass through more atmosphere than normal to reach your eye. Much of the blue light has been scattered out, leaving the red light in a sunset.
Absorption
Different molecules absorb different wavelengths of radiation. For example, O2 and O3 absorb almost all wavelengths shorter than 300 nanometers. Water (H2O) absorbs many wavelengths above 700 nm. When a molecule absorbs a photon, it increases the energy of the molecule. We can think of this as heating the atmosphere, but the atmosphere also cools by emitting radiation, as discussed below.
The combined absorption spectra of the gases in the atmosphere leave "windows" of low opacity, allowing the transmission of only certain bands of light. The optical window runs from around 300 nm (ultraviolet-C) up into the range humans can see, the visible spectrum (commonly called light), at roughly 400–700 nm and continues to the infrared to around 1100 nm. There are also infrared and radio windows that transmit some infrared and radio waves at longer wavelengths. For example, the radio window runs from about one centimeter to about eleven-meter waves.Emission
Objects tend to emit amounts and wavelengths of radiation depending on their "black body" emission curves, therefore hotter objects tend to emit more radiation, with shorter wavelengths. Colder objects emit less radiation, with longer wavelengths. For example, the Sun is approximately 6,000 K (5,730 °C), its radiation peaks near 500 nm, and is visible to the human eye. The Earth is approximately 290 K (17 °C), so its radiation peaks near 10,000 nm, and is much too long to be visible to humans.
The atmosphere emits infrared radiation. For example, on clear nights the Earth's surface cools down faster than on cloudy nights. This is because clouds (H2O) are strong absorbers and emitters of infrared radiation. This is also why it becomes colder at night at higher elevations. The atmosphere acts as a "blanket" to limit the amount of radiation the Earth loses into space.
The greenhouse effect is directly related to this absorption and emission (or "blanket") effect. Some chemicals in the atmosphere absorb and emit infrared radiation, but do not interact with sunlight in the visible spectrum. Common examples of these chemicals are CO2 and H2O. If there are too much of these greenhouse gases, sunlight heats the Earth's surface, but the gases block the infrared radiation from exiting back to space. This imbalance causes the Earth to warm, and thus climate change.
Refractive index
The refractive index of air is close to, but just greater than 1. Systematic variations in refractive index can lead to the bending of light rays over long optical paths. One example is that, under some circumstances, observers onboard ships can see other vessels just over the horizon because light is refracted in the same direction as the curvature of the Earth's surface.
The refractive index of air depends on temperature, giving rise to refraction effects when the temperature gradient is large. An example of such effects is the mirage.
Dimming:
Global dimming is the gradual reduction in the amount of global direct irradiance at the Earth's surface that was observed for several decades after the start of systematic measurements in the 1950s. The effect varies by location, but worldwide it has been estimated to be of the order of a 4% reduction over the three decades from 1960–1990. However, after discounting an anomaly caused by the eruption of Mount Pinatubo in 1991, a very slight reversal in the overall trend has been observed.
Global dimming is thought to have been caused by an increase in particulates such as sulfate aerosols in the atmosphere due to human action. The term sulfate aerosols is used for a suspension of fine solid particles of a sulfate or tiny droplets of a solution of a sulfate or of sulfuric acid (which is not technically a sulfate). They are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception of sea salt sulfate and gypsum dust particles). The two main sulfuric acid precursors are sulfur dioxide (SO2) from anthropogenic sources and volcanoes, and dimethyl sulfide (DMS) from biogenic sources, especially marine plankton. These aerosols can cause a cooling effect on earth.
However the UNFCCC has noted that sulfate aerosols remain in the atmosphere for only a short amount of time in comparison to other greenhouse gases, and therefore their cooling is localized and temporary. Other side effects of sulfate aerosols in the environment include poor air quality. They contribute to acid rain, cause lung irritation, and, according to some sources, have been a main culprit in the depletion of the ozone layer...
Global dimming has interfered with the hydrological cycle by reducing evaporation and may have reduced rainfall in some areas. Global dimming also creates a cooling effect that may have partially masked the effect of greenhouse gases on global warming.
Next article: the atmospheric changes since Earth formed...
the history of the atmosphere...
Picture by Gus... The salinity in Shark Bay is high, due to the place being nearly landlocked and having shallow waters... These salt pans are near "fields" of living stromatolites ...
This article mostly adapted from http://en.wikipedia.org/wiki/Atmosphere_of_Earth and other sources.
Earliest atmosphere
The first gases of the Earth could have been stripped away — and added to — like presently on Mercury, by solar winds early in the history of the planet. Eventually, a state was established in general "not-so-hot cooling" conditions, to become the first atmosphere. Based on today's volcanic evidence, this atmosphere may have contained 60% hydrogen/20% oxygen mostly in the form of water vapour. I have personally estimated the original cloud of water vapour to be around 20,000 kilometres thick in one of the early stages before cooling. I could be wrong on this one...
This atmosphere also had 10% carbon dioxide, 5 to 7% hydrogen sulphide, and smaller amounts of nitrogen, carbon monoxide, free hydrogen, methane and inert gases. A major "rainfall" led to the build-up of a vast ocean, enriched with the other agents in the atmosphere, such as carbon dioxide and later nitrogen plus inert gases. A major part of carbon dioxide was soon dissolved in water and built up carbonate sediments as well as creating some early 'hydrocarbons" in the seas — soon to become enzymes and proteins, the building blocks of life...
Second atmosphere
Water-related sediments have been found dating as early as 3.8 billion years ago. About 3.4 billion years ago, nitrogen was the major part of the then stable "second atmosphere".
Life-forms have to be taken into some account then, even in this early history of the atmosphere, since fossils of early life forms are to be found, dating as early as 3.5 billion years ago. Stromatolite fossils around 3.5 billion years old, found in Australia, show strong evidence of some life being somewhat organised, though primitively, even back then... These early life-forms are thought to be the start of what created the "third" atmosphere, via photosynthesis of mostly blue green algae and micro-organisms...
Stromatolites still exist today in places such as Shark Bay in Western Australia. The high level of salt concentration in the area protects these most ancient surviving-life forms from grazing by other more recent animals.
The geological record however shows a continually relatively warm surface during the complete early temperature record of the Earth, with the exception of one glacial phase about 2.4 billion years ago. This important oxygen-generating process has been developing, though this oxygen was soon reabsorbed by "free" iron, in oxidisation.
Third atmosphere
Plate tectonics, continents constantly rearranged (they are still rearranging today) shaping long-term climate evolution by allowing the transfer of carbon dioxide to large land-based carbonate storages. Free oxygen was mostly held back until about 1.7 billion years ago by iron oxidisation — as shown by the end of the banded iron formations, when more or less, all free "surface" iron had been oxidised...This end signifies a shift from a "reducing" atmosphere to an "oxidising" atmosphere.
Oxygen molecules concentration went up and down until reaching a steady state of more than 15% in the atmosphere. We call the following time span, the Phanerozoic eon, during which oxygen-breathing metazoan life forms began to appear.
Life first "conquered" the hard surface of the planet about 450 million years ago, after having been confined to the seas for more than 3 billion years...
The amount of oxygen in the atmosphere has also gone up and down during the last 600 million years. There was a peak 280 million years ago, when the amount of oxygen was about 30 %. This coincides with the era of the mega-insects such as the meganeura — large insects like dragonflies with wingspan of up to 60 centimetres.... The relationship between insects and oxygen is critical. Insects breathe through their carapace/skin and cannot grow beyond a certain size in accordance with the ratio of oxygen in the air.
Periods with more oxygen in the atmosphere are believed to cause rapid development of animals. Today, due to nearly 21 percent oxygen, this period is still regarded as encouraging the rapid development of animals... Note: oxygen is viewed as a "cooling gas" in the "greenhouse effect" equation... Nitrogen is neither here nor there...
Please not that most of these transformation happened in the lower part (10,000 metres thick from the surface) which contains about 75 % of the molecules of the atmospheric gases.
-----------------------------
Two main processes still change the atmospheric composition of gases:
Plants converts carbon dioxide into the bodies of the plant, which release oxygen into the atmosphere via photosynthesis.
The break down of pyrite rocks cause sulphur to be added to the oceans. Volcanos cause this sulphur to be oxidised, reducing some oxygen in the atmosphere. But volcanos also emit some carbon dioxide, so that plants can convert it into oxygen. But the amount of CO2 available is somewhat limited. The cause of the variation of oxygen in the atmosphere is not fully-known but can be attributed to period of cool and warm from the sun, as well as general "greenhouse effect" of specific time and/or volcanic activity...
These two processes have been the main stay of the carbon equation for the last few million years with relatively small changes to its equilibrium — in which atmospheric CO2 concentration has been oscillating between 100 to 200 ppm...
Currently, anthropogenic greenhouse gases (CO2, methane and a few others) are increasing in the atmosphere. Increases such as this are paralleled with previous period of recorded global warming.
Since the industrial revolution, in 1850, humans have added about 245 ppm of carbon dioxide to the 150 measured back then — the level of CO2 being 395 ppm presently.
Scientists, aware of all the atmospheric gas composition and of previous instances of global warming (including that at the end of the last ice age 12,000 years ago) — rather than being plain meteorologists who concentrate on air-mass movements — are VERY ALARMED at this large increase. This important proportional increase CANNOT reabsorbed by plants nor by the oceans (now approaching CO2 saturation and turning "acidic") thus the increase in the level of CO2 parallels irrevocably past increases in temperature of the lower atmosphere.
--------------------------
How have humans changed the CO2 proportion in the atmosphere?
Humans are burning "fossil fuels" (from carbon sinks/storage) that have buried for aeons under bedrock in the form of coal, oil and gas... Humans have thus added more than twice the amount in extra carbon into this long-standing equilibrium of plant/sea/animals/atmospheric CO2.
Plus other forms of air pollution
Air pollution is the introduction of chemicals, particulate matter, or biological materials — that cause harm or discomfort to organisms —into the atmosphere. Stratospheric ozone depletion is believed to be caused by air pollution (chiefly from chlorofluorocarbons).
Next: The Carbon Cycle — including photosynthesis...
the surface carbon cycle
picture by Gus leonisky — bug on a cabbage leaf.
Until now, humans have not been able to replicate or find an equivalent to the dismantling of the carbon dioxide molecule that plants are doing daily...
CO2 is a very stable molecule and difficult to pull apart without spending more energy than CO2 itself can radiate.
Photosynthesis is a complex process that using light (photons) and bio-chemistry to take away the carbon atom to build plant cells, including cellulose.
From Wikipedia http://en.wikipedia.org/wiki/Photosynthesis
Photosynthesis is a process used by plants and other organisms to capture the sun's energy to split off water's hydrogen from oxygen.
Hydrogen is combined with carbon dioxide (absorbed from air or water) to form glucose and release oxygen.
All living cells in turn use fuels derived from glucose and oxidize the hydrogen and carbon to release the energy and reform water and carbon dioxide in the process (cellular respiration ‚ see also ATP).
Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea (animal kingdom). Photosynthetic organisms are called photoautotrophs, since they can "create their own food". In plants, algae, and cyanobacteria, photosynthesis uses carbon dioxide and water, releasing oxygen as a waste product.
Photosynthesis is vital for all aerobic life on Earth.
In addition to maintaining normal levels of oxygen in the atmosphere, photosynthesis is the source of energy for nearly all life on earth, either directly, through primary production, or indirectly, as the ultimate source of the energy in their food, the exceptions being chemoautotrophs that live in rocks or around deep sea hydrothermal vents.
The rate of energy capture by photosynthesis is immense, approximately 100 terawatts, which is about six times larger than the power consumption of human civilization.
As well as energy, photosynthesis is also the source of the carbon in all the organic compounds within organisms' bodies. In all, photosynthetic organisms convert around 100–115 petagrams of carbon into biomass per year.
Although photosynthesis can happen in different ways in different species, some features are always the same.
The process always begins when energy from light is absorbed by proteins called photosynthetic reaction centers that contain chlorophylls. In plants, these proteins are held inside organelles called chloroplasts, while in bacteria they are embedded in the plasma membrane.
Some of the light energy gathered by chlorophylls is stored in the form of adenosine triphosphate (ATP).
The rest of the energy is used to remove electrons from a substance such as water.
These electrons are then used in the reactions that turn carbon dioxide into organic compounds.
In plants, algae and cyanobacteria, this is done by a sequence of reactions called the Calvin cycle, but different sets of reactions are found in some bacteria, such as the reverse Krebs cycle in Chlorobium.
Many photosynthetic organisms have adaptations that concentrate or store carbon dioxide. This helps reduce a wasteful process called photorespiration that can consume part of the sugar produced during photosynthesis.
CO2 + 2n DH2 + photons → 2(CH2O)n + 2n DOCarbon dioxide + electron donor + light energy → carbohydrate + oxidized electron donor
In oxygenic photosynthesis water is the electron donor and, since its hydrolysis releases oxygen, the equation for this process is:
2n CO2 + 4n H2O + photons → 2(CH2O)n + 2n O2 + 2n H2Ocarbon dioxide + water + light energy → carbohydrate + oxygen + water
Often 2n water molecules are cancelled on both sides, yielding:
2n CO2 + 2n H2O + photons → 2(CH2O)n + 2n O2carbon dioxide + water + light energy → carbohydrate + oxygen
Other processes substitute other compounds (such as arsenite) for water in the electron-supply role; the microbes use sunlight to oxidize arsenite to arsenate:[11] The equation for this reaction is:
CO2 + (AsO33–) + photons → (AsO43–) + CO[12]carbon dioxide + arsenite + light energy → arsenate + carbon monoxide (used to build other compounds in subsequent reactions)
-----------------------
The Food chain....
Without photosynthesis the "Earthian" food chain would be non-existent. Without photosynthesis, there would be little bacteria (no mitochindrion in animals), no plants, no herbivores, no insects, no omnivores.
Up to 150 years ago the photosynthetisation of CO2, the absorption of CO2 by the oceans and the CO2 content of the atmosphere was in a "carbon equation balance" at between 100 to 200 ppms CO2 of the gaseous composition of the atmosphere.
Presently, the human added CO2 from the burning of fossil fuel is not fully absorbed by the oceans nor by the enormous ability of photosynthesis. A residual 3ppm of CO2 from human activity is added every year in the atmosphere... Furthermore, we have deforested the planet substantially and replaced the trees with crops that are not as carbon capturing efficiently as trees...
The net gain is an increase of CO2 in the atmosphere from human activity.
Next: factors that cool and warm the atmosphere
Of global warming and cooling
Picture by a friend of Gus — flying at the tropopause level. Below one can see cultivated fields, above one can see the stratosphere and the "beyond" we call space.
There are 7 main factors that impact on the cooling and warming of the surface of the earth and the immediate atmosphere above it:
Climatic oscillations,
Solar activity,
Volcano activity,
Gaseous mix,
Dimming, Colour
Bolides (cosmic events),
Life: plants absorption of CO2 and release of O2 — animals absorption of O2 and release of CO2 — Blue green algae release of oxygen (and toxins)... Permafrost and decay of life release of methane.
CLIMATIC OSCILLATIONS
A) Wobbles of the Earth axis
some glaciation events have been attributed to such — when other conditions are favourable but not necessarily enough...
B) Climatic patterns such as La Nina and El Nino
El Nino generally leads to higher temperature average for the surface of the planet
La Nina generally leads to cooler temperature average for the surface of the planet
C) other oscillations that have local impact on weather patterns.
SOLAR ACTIVITY
Solar flares and sun spots generally lead to higher temperature average for surface of the planet
low sun activity generally leads to cooler temperature average for the surface of the planet
GASEOUS MIX
More CO2 generally leads to higher temperature average for surface of the planet
Less CO2 generally leads to cooler temperature average for the surface of the planet
More oxygen generally leads to cooler temperature average for the surface of the planet
Less oxygen generally leads to higher temperature average for the surface of the planet
More clear water vapour generally leads to higher temperature average for surface of the planet
More cloudy water vapour can lead to lower temperature average for surface of the planet by dimming More methane leads to higher temperature average for the surface of the planet Sulphur dioxide leads to cooler temperature average for the surface of the planet Man made aerosols can induce dimming that can lead to slightly cooler temperature
BOLIDES
Comets and meteorites impacts can lead to lower or higher temperatures on the Earth depending on many factors.
VOLCANIC ACTIVITY
Volcanos can release both sulphur dioxide and carbon dioxide but their plume of ash, steam and general cloud size can induce noticeable dimming that leads to some cooling.
-----------------------------
PRESENT TRENDS
Despite the denialists song and dance, 99.9 per cent of scientists — including sceptic scientists who challenge the anthropomorphic origin of warming — agree that the surface of the planet is warming by around 0.03 degree Celsius per annum or 0.3 degree Celsius per decade or 3 degrees per one hundred years — THIS FIGURE IS CONSIDERED AS A MINIMUM
This figure does not include the compounding effect of increase temperature, nor the possibility of warming feed back mechanisms — such as massive increase of methane by modest warming.
This "small" increase of 0.03 per annum is not noticeable to mere mortals. Only precise instrument measurements can define this warming trend on such a global scale. Instrumentation calibration is critical.
Such small increase of temperature represents an enormous EXTRA amount of "energy" being loaded into and being dissipated by the Atmosphere... This trend has had an impact on weather patterns and weather extremes though one is still cautious in apportioning the blame of such "noticeable" present changes.
Some weather changes are quite weird:
Cold wet winter in the US, unusually warmer conditions near the arctic...
Hot dry conditions that lead to "never seen" fires in Australia (record temperature and wind 2010) and the US (2012);
Record hot Siberian temperature above 39 degree Celsius last year. Melting of the permafrost.
Very humid conditions leading to "floods of the century" every decade or more frequently...
The melting of glaciers, which in itself absorbs a lot of heat energy and retards the global warming trend.... Some glaciers are "growing" in specific areas— such areas are cold and "getting wetter" that previously recorded — such as the centre of Antarctica (warming up nonetheless) and some part of the Himalayas.
Higher average sea surface temperatures.
World sea level has risen by about 15 centimetres since 1850 — this contentious figure is accepted by the insurance industries and reinsuring bodies... The insurance industry use empirical data based on all claims made, claims that are increasing sharply due to "more frequent natural" disasters.... (One needs to appreciate the insurance industry views. For example, premiums for drivers under 25 years of age are about twice that of normal premiums because the empirical data suggest that these drivers will have three times more accidents than above 25 drivers...)
--------------------------------
What can induce the present trend of warming:
EARTH AXIS WOBBLES
in average position — no effect to take note of.
CLIMATIC OSCILLATION:
In the last couple of years, La Nina (lasting a bit longer than usual) has NOT made a dent in the warming of the surface of the planet.
Other climatic oscillation have made impacts on local conditions but the average is still trending to warming.
VOLCANIC ACTIVITY:
Krakatoa in Indonesia (1883) had a marked influence in cooling the temperature tend
Mount Pinatubo in Indonesia (1991) created a small cooling worldwide
Iceland's Eyjafjallajokull Gigjokull volcano (2010) had no noticeable effect on cooling or warming
---------------------------------
The full list of the LARGEST recent eruptions: (Mount St Helens in the US does not rate) since 1258
Mount Pinatubo Luzon Volcanic Arc 1991, June 15
Novarupta Aleutian Range 1912, June 6
Santa María Central America Volcanic Arc 1902, Oct 24
Mount Tarawera Taupo Volcanic Zone 1886, Jun 10
Krakatoa Sunda Arc 1883, Aug 26-27
Mount Tambora Lesser Sunda Islands 1815, Apr 10
Grímsvötn and Laki Iceland 1783-85
Long Island (Papua New Guinea) Bismarck Volcanic Arc 1660
Kolumbo, Santorini South Aegean Volcanic Arc 1650, Sep 27
Huaynaputina Andes, Central Volcanic Zone 1600, Feb 19
Billy Mitchell Bougainville & Solomon Is. 1580
Bárðarbunga Iceland 1477
1452-53 ice core event New Hebrides Arc 1452-53
Andes, Northern Volcanic Zone 1280
Rinjani Lombok, Lesser Sunda Islands 1258
---------------------
Little Ice Age (1350-1850)
Evidence from mountain glaciers does suggest increased glaciation in a number of widely spread regions outside Europe prior to the 20th century, including Alaska, New Zealand and Patagonia. However, the timing of maximum glacial advances in these regions differs considerably, suggesting that they may represent largely independent regional climate changes, not a globally-synchronous increased glaciation. Thus current evidence does not support globally synchronous periods of anomalous cold or warmth over this time frame, and the conventional terms of "Little Ice Age" and "Medieval Warm Period" appear to have limited utility in describing trends in hemispheric or global mean temperature changes in past centuries... Hemispherically, the "Little Ice Age" can only be considered as a modest cooling of the Northern Hemisphere during this period of less than 1°C relative to late 20th century levels.
Several causes have been proposed: cyclical lows in solar radiation, heightened volcanic activity, changes in the ocean circulation, an inherent variability in global climate, or decreases in the human population (plague and such). Lower CO2 atmospheric concentrations as noticed in Antarctic ice cores for this period may have also resulted from (or created) the colder global climate.
--------------------------------
SOLAR ACTIVITY:It has been noted that the sun has been "dormant" for the past 11 years. Yet six of the ten warmest years since 1850 have happened in the last decade. It has been noticed too that the Thermosphere has been cooling down to 1100 from 1400 degree Celsius in the past decade — YET the lower atmosphere has still been warming up.
Presently the sun is "waking up" and likely to increase the general temperature of the planet slightly.
--------------------------
GASEOUS MIX:
Presently CO2 has more than doubled its level in the atmosphere since 1850 AND RISING.
Most of this can be attributed to anthropomorphic activities.
The amount of CO2 released by human activities can be easily calculated by knowing how much fossil fuel we consume and transform into CO2... From steel making to transport we burn the carbon in coal and oil and produce about 5 to 6 ppms of carbon dioxide (CO2) added into the atmosphere. Some of this (up to 30 per cent being reabsorbed by the oceans) is processed by the "natural carbon cycle" but about 3 ppms per year REMAINS as EXTRA CO2 in the atmosphere. Since 1850, the anthropomorphic amount of CO2 added is now approaching more than 240 ppms.
DIMMING, COLOUR
The more clouds (opaque, white water vapour), the less heat from the sun can penetrate down to the surface.
But, in reverse, the less heat can escape from the surface back into space.
Meanwhile, the more heat, the more likely to have clear water vapour depending on the dew point. Thus the dynamics of this complex process can interferred with a straight expected warming by giving strong periods of warming and some periods of cooling,.
Nonetheless, the trend over several years is going towards warming.
Colour: A black surface will warm faster than a white surface, This is a somewhat important part of the equation which leads say to the arctic region to become warmer because it can absorb more heat from the sun instead of reflecting it, as the ice melts.
Next: what does this means and should we worry...
Are we human enough?
Low unshapen cloud over the city of Sydney... Picture by Gus.
We now know that humans are producing more CO2 than the "natural carbon cycle" can "digest".
We know that temperatures and CO2 are linked in some way.
We know that CO2 is a "greenhouse" gas.
We know that methane is a very "greenhouse" gas...
We know that clear water vapour is a "greenhouse" gas
We know that there is no present volcanic activity large enough to interfere with the climate...
Aerosols that used to destroy the ozone layer have been banned.
We know La Nina is getting weak, being replaced by a warmer El Nino
We know that the sun is entering a more active cycle
We know that glaciers are melting faster
We know that the temperature trend is going up
We know the arctic ice is diminishing
We know that at the Tropopause tropical temperatures (in the minus 55s) have climbed 0,6 degree Celsius per decade since the 1970.
We know that strong extreme climatic events have become more frequent
We know that the sea level has risen at least 15 centimetres since 1850.
-------------------------------------
Are we human enough to take the leap and link CO2 as the main culprit to this "change"?
Svante Arrhenius took this leap 123 years ago. Svante may prove more important than Albert (Einstein) in our practical survival on this planet...
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction.[ The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish chemist Svante Arrhenius provided a physical justification and interpretation for it. Currently, it is best seen as an empirical relationship.[2] It can be used to model the temperature-variance of diffusion coefficients, population of crystal vacancies, creep rates, and many other thermally-induced processes/reactions.
A historically useful generalization supported by the Arrhenius equation is that, for many common chemical reactions at room temperature, the reaction rate doubles for every 10 degree Celsius increase in temperature.
Arrhenius developed a theory to explain the ice ages, and in 1896 he was the first scientist to speculate that changes in the levels of carbon dioxide in the atmosphere could substantially alter the surface temperature through the greenhouse effect.[4] He was influenced by the work of others, including Joseph Fourier. Arrhenius used the infrared observations of the moon by Frank Washington Very and Samuel Pierpont Langley at the Allegheny Observatory in Pittsburgh to calculate the absorption of infrared radiation by atmospheric CO2 and water vapour. Using 'Stefan's law' (better known as the Stefan Boltzmann law), he formulated his greenhouse law. In its original form, Arrhenius' greenhouse law reads as follows (wikipedia):
if the quantity of carbonic acid increases in geometric progression, the augmentation of the temperature will increase nearly in arithmetic progression.This simplified expression is still used today:
ΔF = α ln(C/)Arrhenius' high absorption values for CO2, however, met criticism by Knut Ångström in 1900, who published the first modern infrared spectrum of CO2 with two absorption bands. Arrhenius replied strongly in 1901 (Annalen der Physik), dismissing the critique altogether. (Gus: the experiment by Ångström was too small to be conclusive)
Arrhenius touched the subject briefly in a technical book titled Lehrbuch der kosmischen Physik (1903). He later wrote Världarnas utveckling (1906), German translation: Das Werden der Welten (1907), English translation: Worlds in the Making (1908) directed at a general audience, where he suggested that the human emission of CO2 would be strong enough to prevent the world from entering a new ice age, and that a warmer earth would be needed to feed the rapidly increasing population:
Do I trust Arrhenius solid mathematical analysis of chemistry and physics, or do I follow one of Jo Nova's stupid rants?... The choice is mine and yours... Jo Nova's rant is a worry... though I am sceptic of Arrhenius' philosophical interpretation for the future of mankind...
One has to realise that until the mid 1940s, many scientist working on long range climate progression were of the conviction that the Earth was about to enter a new ice age, or a mini-ice age at least. It was not a secret, I remember my father talking about it.
But by the early 1950s, things were not going according to plan, even after some harsh European winters in the late 1940s... Something was warming up the atmosphere...
------------------------
So what can we expect from a global warming of say 2 degrees Celsius by 2100?...
Though it is difficult to predict with high degree of precision, one can expect an increase of clear water vapours and cloudy water vapours leading to greater differential stress in the immediate atmosphere thus more damaging storms.
We can expect a sea level rise of 45 centimetres minimum.
We can expect "snap" hot droughts and violent floods.
We can expect a greater change of climatic zone banding (as has been noticed already). Extension of tropical bands, squeezing the Temperate zones into the "warmer" and more climatically stressed polar zones.
Present ocean currents may shift the position of their "turning points" where water sinks to the bottom or where water rise to the surface. Some computer models predict the slowing of the gulf stream, leading to a colder Western Europe, with stronger weather eddies (cyclonic winds and conditions) on the edge of temperate and sub tropical-tropical bandings.
Some of the denialists are suggesting that Coral Reefs would grow better as presently recorded south of Japan as the seas are getting warmer... But Coral Reefs in already warm water, such as the Great Barrier Reef, could be stressed beyond survival north of a line above Bundaberg. The CO2 dissolved in the sea also makes the water acidic and has an impact on the development of many species... Other factor such as pollutants and fertiliser also have an impact.
Temperature of 49 degree Celsius could be recorded in Victoria, creating "vicious firestorms when associated with strong winds"... Stronger monsoon in the north of Australia and more violent cyclones landing near Cairns... More violent flash foods in Europe, more wild-fires everywhere.
Wild fires can dim the sunlight but this dimming is cancelled by the CO2 and the soon to be clear-water vapour they produce...
All this will become noticeable by "1998". By 2015, things will take a leap for the worse and by 2032 (2030-2035) it ain't going to look pretty.
----------------------------
But what one has to realise is that two (2) degrees is only the MINIMUM extra temperature by 2100 — on the trends calculated if we act upon our emissions of CO2...
That is to say, we need to cut our CO2 emissions by 50 per cent based on 1998 levels, by 2050 (not even forty years from now) and by 75 per cent by 2070.
With a human population becoming more and more hungry for energy, food and water (It has been calculated that in the next 40 years, humans will consume more food than has been consumed in the past 8000 years), we are facing a Herculean task.
Thus should we do something?...
Burn baby burn...
I know some pragmatic rich people have taken the simple view that even if global warming was coming at us at full bore, there is nothing we can do. Thus these rich pragmatists are preparing for wars, preparing for massive sea rise and horrendous climatic conditions...
Should we do nothing, the temperature rise is likely to be around 6 degree Celsius by 2100 with massive consequences, including a sea level rise of more than 4 metres. In the meantime, enjoy life...
Note that, at the extreme scale of possible global temperatures with the present settings plus with change in CO2 level using all the stored carbon sinks, seas can go as high as 75 metres above present level still, should most of the general conditions be there...
But according to my calculations, one of the important condition (continental position — the southern ocean) is not met thus the sea level rise would be limited to only half of that (35 metres)...
We are sleeping in the bed we are making — our nightmare has not begun yet...
the sun burps...
This post was originally published on Mashable.
From here on Earth, the Sun looks remarkably consistent, continuously bathing our planet in light and heat without much change from year to year, let alone day to day.
In reality, it's anything but calm, as this incredible NASA photo of a coronal mass ejection (CME) shows.
Read more: http://www.smh.com.au/technology/sci-tech/nasa-captures-epic-sun-burp-20120906-25gdg.html#ixzz25fN3kAsX
As I have mentioned before, in 1859, there was a solar flare that disrupted a lot of the electricity supply around the world... Apparently should a similar event occur now, the bill for the damage would be around 10 trillion dollars...
On September 1, 1859, British astronomer Richard Carrington saw something extraordinary: amidst the usual shifting sunspots his telescope projected onto a sheet of paper, several blobs of blindingly white light grew and faded over the space of five minutes. His sketch is the earliest record of a solar flare, a rare"white light" solar flare.
The next day, the charged plasma flung out by the sun reached Earth. It lit up the entire northern hemisphere, right down to Hawaii and Rome, with vivid red, blue, green auroras. The spectacular display was covered in numerous newspaper reports, which could actually be read at night by the glow. There were also reports of magnetic disturbances: compasses went haywire during the bombardment.
More seriously, the solar storm battered the world's infant communication network. Telegraph wires burst into flames, touching off fires (while in other cases fire crews were called to fires that did not exist, due to the fiery lights in the sky). Telegraph machines scorched paper printouts, stunned operators with electric shocks, transmitted gibberish, and continued working for hours even after being unplugged from the batteries that powered them. The Earth itself was no longer "grounded"!
For two days, the light show and electromagnetic storm continued, then faded.
http://greekgeek.hubpages.com/hub/massive-solar-flare-1859
I would not be surprised if the recent event in August 31 (very close to september 1 — that reached the earth till september 3rd) was not at the origin of some computer black outs in town... Mine went funny with sparks on the modem and I know of another computer that stopped working for a few days then worked again without any geek understanding the why of the matter...
Beautiful images mind you...
a message from those who flew higher than the crow...
https://www.youtube.com/watch?v=NN1eSMXI_6Y
Read from top....
sun is going quiet...
The Sun has gone blank, scientists say.
For the second time in less than a month, not a single dark sunspot can be seen on the surface of our star.
It's a sign that the Sun is entering a new stage in its solar cycle, a phase of decreased activity.
This particular period of sunspotlessness will last for a few days. But eventually the periods will span for weeks and then perhaps months as the Sun goes into "solar minimum".
Our Sun is a volatile star, and its surface is marked with sunspots. Sunspots, the dark areas on the Sun's surface which can be seen without a telescope, are areas of intense magnetic activity, which reduce the temperature of the star in their vicinity.
They can prompt solar flares, radiation storms and geomagnetic storms here on Earth, causing phenomena such as the aurora australis (southern lights) and aurora borealis (northern lights).
The Sun's surface changes through 11-year solar cycles. The greatest number of sunspots in any given solar cycle is the "solar maximum." The lowest number is "solar minimum".
According to NASA, "during Solar Max, huge sunspots and intense solar flares are a daily occurrence. Auroras appear in Florida. Radiation storms knock out satellites. Radio blackouts frustrate CB radio as well. The last such episode took place in the years around 2000-2001.
"During solar minimum, the opposite occurs. Solar flares are almost non-existent while whole weeks go by without a single, tiny sunspot to break the monotony of the blank sun. This is what we are experiencing now."
So now, as our star enters a solar minimum period, the sunspots will slowly disappear. But that doesn't mean it's all going to go quiet in space.
History shows there can still be big events during weak solar cycles. And, if the activity is strong enough, it could threaten our technology, wiping out electricity, the internet and jamming satellite signals across entire continents.
In 1859, during a low point in the solar cycle, the "Carrington Event" prompted geomagnetic storms as far south as the Caribbean. Another event of that magnitude could hugely disrupt our lives today.
What's next?
The last few cycles have been getting progressively weaker, with fewer sunspots being produced in each successive cycle. It's too early to know if the 2020 cycle will continue this trend. The Sun isn't due to hit its solar maximum again until midway through the 2020s.
But space weather forecasters - the people whose job it is to try and predict and protect us from the impacts of solar events - are already looking to that next cycle.
Read more: http://www.smh.com.au/technology/sci-tech/why-the-sun-has-lost-its-spots--and-what-that-means-for-us-20160629-gpv1b0.html#ixzz4D1TOsRjN
Follow us: @smh on Twitter | sydneymorningherald on Facebook
Read from top....